Solution: Hybridization is introduced to explain the geometry of bonding orbitals in valance bond theory. Give the shape that describes each hybrid orbital set: (a) sp2 (b) sp3d (c) sp (d) sp3d2 Explain why a carbon atom cannot form five bonds using sp3d hybrid orbitals. Solution: There are no d orbitals in the valence shell of carbon.
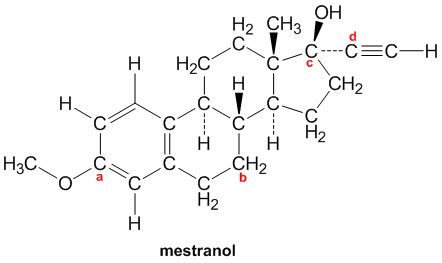
Solved 2. Label the hybridization of each carbon atom in the | Chegg.com
Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in Org 1. For a given atom: Count the number of atoms connected to it (atoms – not bonds!) Count the number of lone pairs attached to it. Add these two numbers together. If it’s 4, your atom is sp3.
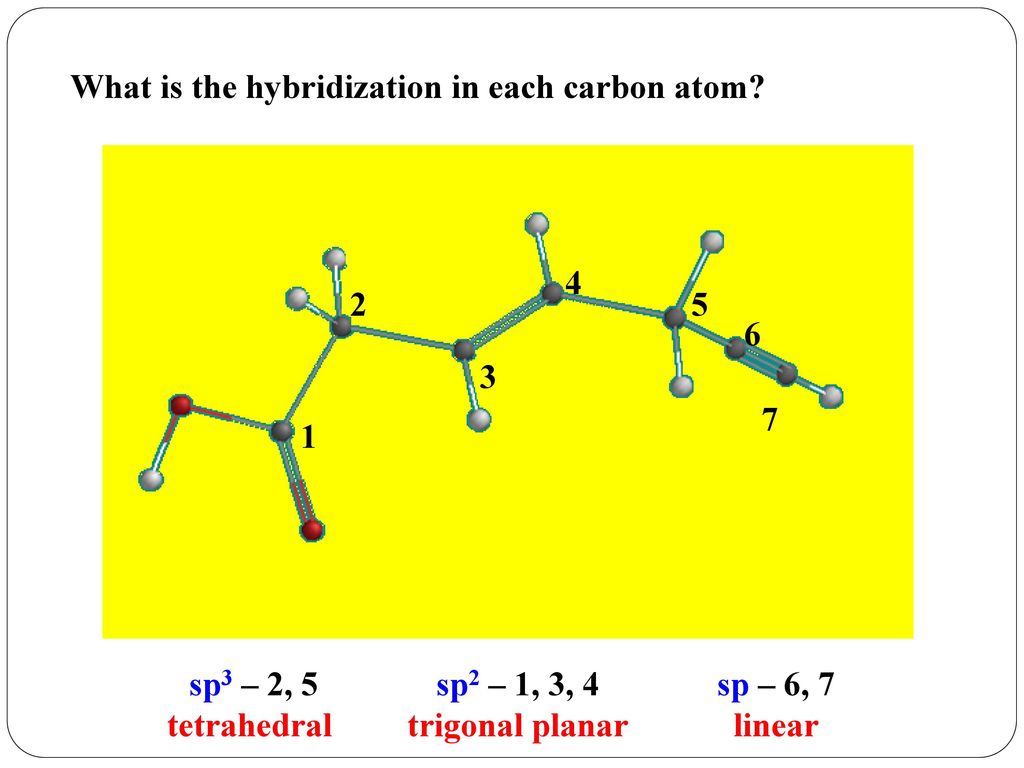
Source Image: slideplayer.com
Download Image
ALKENES AND sp 2 HYBRIDIZATION OF CARBON. We will now reproduce the sp 3 hybridization process for carbon, but instead of taking one s and three p orbitals to make four equivalent sp 3 orbitals, this time we’ll take only one s and two p orbitals to make three equivalent sp 2 orbitals, leaving one p orbital untouched. The process is shown below. As shown, the three resulting sp 2 orbitals are
Source Image: chegg.com
Download Image
Solved lewis structure for a molecule of tartaric acid. a. | Chegg.com Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon’s 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron
Source Image: chegg.com
Download Image
Label Each Carbon Atom With The Appropriate Hybridization.
Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon’s 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron Each CH bond in methane, then, can be described as an overlap between a half-filled 1s orbital in a hydrogen atom and the larger lobe of one of the four half-filled sp 3 hybrid orbitals in the central carbon. The length of the carbon-hydrogen bonds in methane is 1.09 Å (1.09 x 10-10 m).
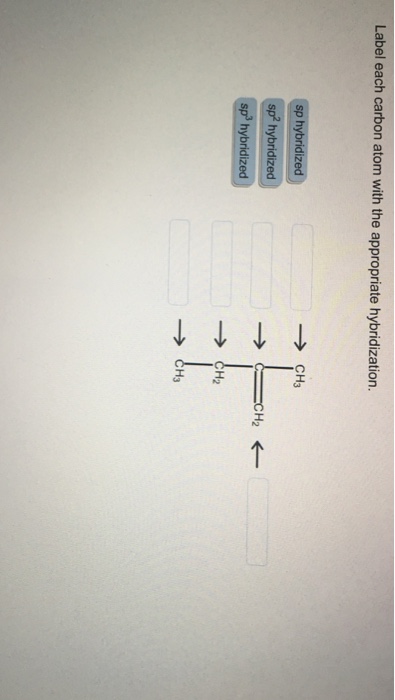
Solved Determine the hybridization whether its sp3 sp2 or sp | Chegg.com
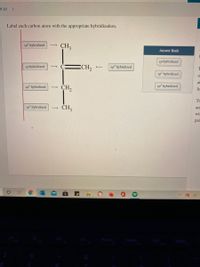
Label each carbon atom with the appropriate hybridization. Verified Solution. 2m. Play a video: … Previous problem. Next problem. 0:51 m. Watch next. Master Hybridization Concept 1 with a bite sized video explanation from Jules Bruno. Start learning. Comments (0) Related Videos. Related Practice. Guided course. 00:51. Hybridization Concept 1 Answered: of 20 > Label each carbon atom with the… | bartleby
 Download Image
Download ImageSOLVED: Label each carbon atom with the appropriate hybridization. CH Answer Bank sp hybridized CH2 hybridized CH hybridized CH3 Label each carbon atom with the appropriate hybridization. Verified Solution. 2m. Play a video: … Previous problem. Next problem. 0:51 m. Watch next. Master Hybridization Concept 1 with a bite sized video explanation from Jules Bruno. Start learning. Comments (0) Related Videos. Related Practice. Guided course. 00:51. Hybridization Concept 1
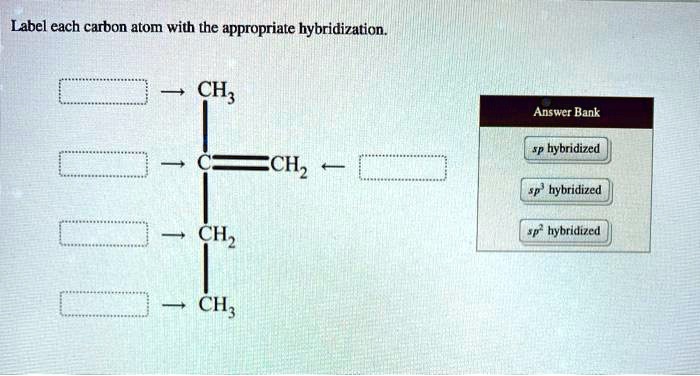
Source Image: numerade.com
Download Image
Solved 2. Label the hybridization of each carbon atom in the | Chegg.com Solution: Hybridization is introduced to explain the geometry of bonding orbitals in valance bond theory. Give the shape that describes each hybrid orbital set: (a) sp2 (b) sp3d (c) sp (d) sp3d2 Explain why a carbon atom cannot form five bonds using sp3d hybrid orbitals. Solution: There are no d orbitals in the valence shell of carbon.

Source Image: chegg.com
Download Image
Solved lewis structure for a molecule of tartaric acid. a. | Chegg.com ALKENES AND sp 2 HYBRIDIZATION OF CARBON. We will now reproduce the sp 3 hybridization process for carbon, but instead of taking one s and three p orbitals to make four equivalent sp 3 orbitals, this time we’ll take only one s and two p orbitals to make three equivalent sp 2 orbitals, leaving one p orbital untouched. The process is shown below. As shown, the three resulting sp 2 orbitals are
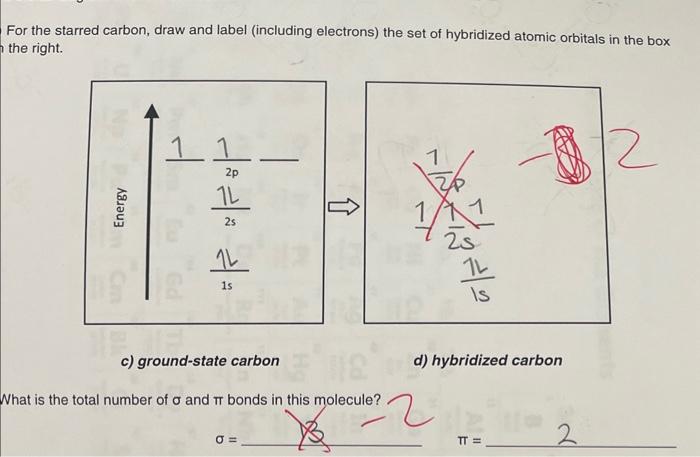
Source Image: chegg.com
Download Image
Aleks Identifying carbon hybridization in simple organic molecules – YouTube We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Created by Jay. Questions Tips & Thanks Want to join the conversation? Sort by:

Source Image: m.youtube.com
Download Image
SOLVED: Label each carbon atom with the appropriate hybridization. CH Answer Bank sp hybridized CH2 hybridized CH hybridized CH3 Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon’s 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron

Source Image: numerade.com
Download Image
Answered: Identifying carbon hybridization in… | bartleby Each CH bond in methane, then, can be described as an overlap between a half-filled 1s orbital in a hydrogen atom and the larger lobe of one of the four half-filled sp 3 hybrid orbitals in the central carbon. The length of the carbon-hydrogen bonds in methane is 1.09 Å (1.09 x 10-10 m).
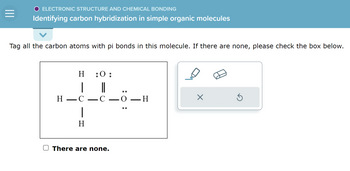
Source Image: bartleby.com
Download Image
SOLVED: Label each carbon atom with the appropriate hybridization. CH Answer Bank sp hybridized CH2 hybridized CH hybridized CH3
Answered: Identifying carbon hybridization in… | bartleby Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in Org 1. For a given atom: Count the number of atoms connected to it (atoms – not bonds!) Count the number of lone pairs attached to it. Add these two numbers together. If it’s 4, your atom is sp3.
Solved lewis structure for a molecule of tartaric acid. a. | Chegg.com SOLVED: Label each carbon atom with the appropriate hybridization. CH Answer Bank sp hybridized CH2 hybridized CH hybridized CH3 We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Created by Jay. Questions Tips & Thanks Want to join the conversation? Sort by: