Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals.
Difference Between Molecules And Compounds In Tabular Form
3.1 I can compare and contrast the structure of ionic and covalent compounds and give examples of each. 3.2 I can determine the number of atoms in a compound when given the formula. 3.3 I can name ionic compounds when given the formulas. 3.4 I can write formulas for ionic compounds when given the names.

Source Image: study.com
Download Image
Sep 19, 2022Nomenclature, a collection of rules for naming things, is important in science and in many other situations.This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3, and N 2 O 4.The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions

Source Image: pubs.acs.org
Download Image
SOLVED: Contrast the structures of ionic compounds and metals.
(a) only elements in the same row of the periodic table (b) only elements in the same column of the periodic table (c) elements with significant differences in electronegativity (d) elements with
Source Image: numerade.com
Download Image
Contrast The Structure Of Ionic Compounds And Metals
(a) only elements in the same row of the periodic table (b) only elements in the same column of the periodic table (c) elements with significant differences in electronegativity (d) elements with
Ionic compounds form crystal lattices rather than amorphous solids. They have higher enthalpies of fusion and vaporization than molecular compounds. They are hard. They are brittle. They have high melting points and also high boiling points. They conduct electricity but only when they are dissolved in water. … Deevona · 52 · Oct 2 2014 Questions
⏩SOLVED:Contrast the structures of ionic compounds and metals. | Numerade
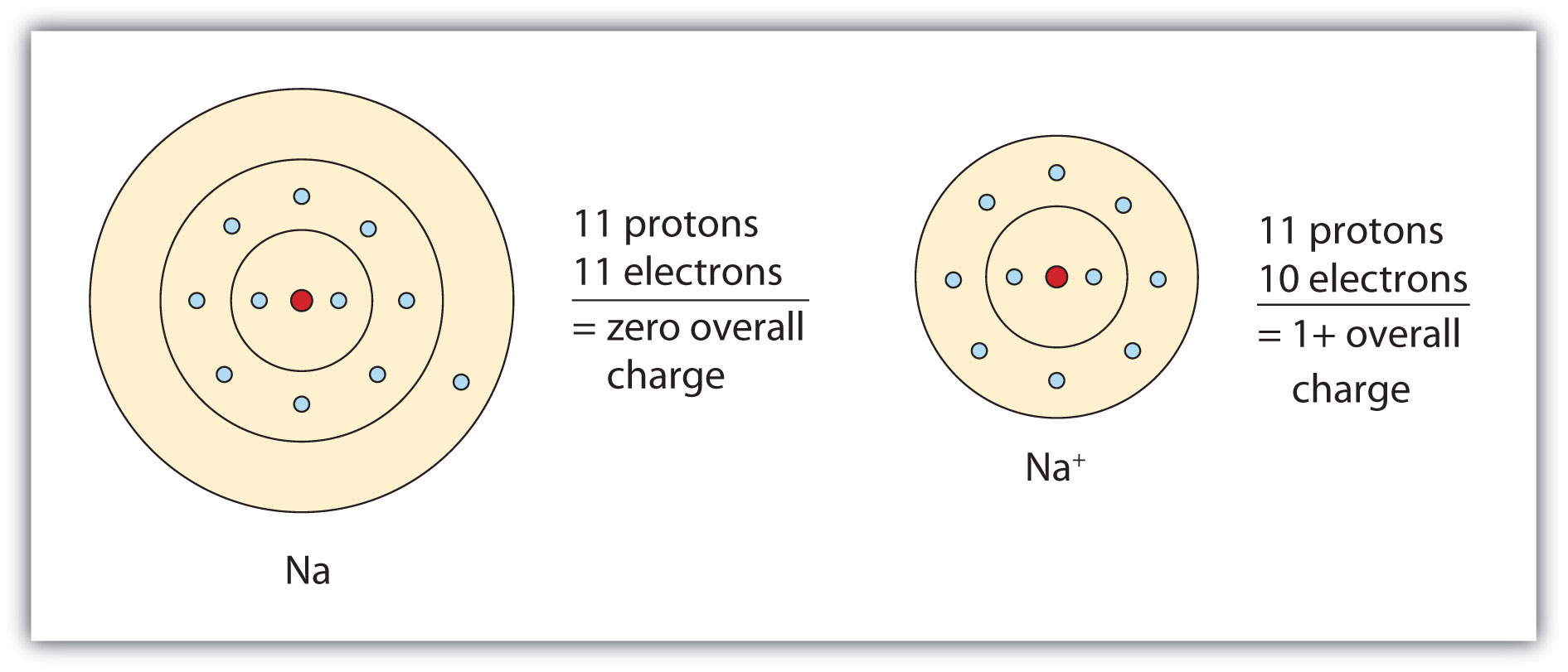
Ionic compounds have alternating charges, which means that they are composed of oppositely charged ions (positively charged refers to cations and negatively charged refers to anions) that are held together by an ionic bond, while metals can form only cations which are surrounded by a sea of mobile or delocalized electrons, due to its properties, so metals are a participant in an ionic compound
CH103 – CHAPTER 4: Ions and Ionic Compounds – Chemistry
Source Image: wou.edu
Download Image
Electrical Double Layer Structure in Ionic Liquids and Its Importance for Supercapacitor, Battery, Sensing, and Lubrication Applications | The Journal of Physical Chemistry C
Ionic compounds have alternating charges, which means that they are composed of oppositely charged ions (positively charged refers to cations and negatively charged refers to anions) that are held together by an ionic bond, while metals can form only cations which are surrounded by a sea of mobile or delocalized electrons, due to its properties, so metals are a participant in an ionic compound

Source Image: pubs.acs.org
Download Image
Difference Between Molecules And Compounds In Tabular Form
Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals.

Source Image: toppr.com
Download Image
SOLVED: Contrast the structures of ionic compounds and metals.
Sep 19, 2022Nomenclature, a collection of rules for naming things, is important in science and in many other situations.This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3, and N 2 O 4.The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions
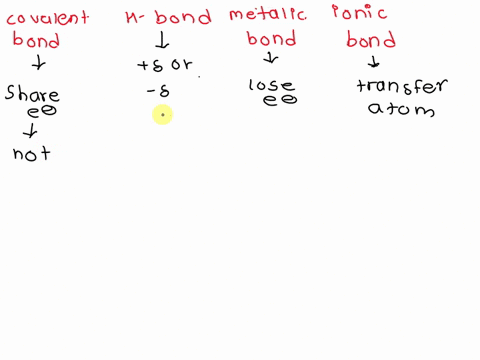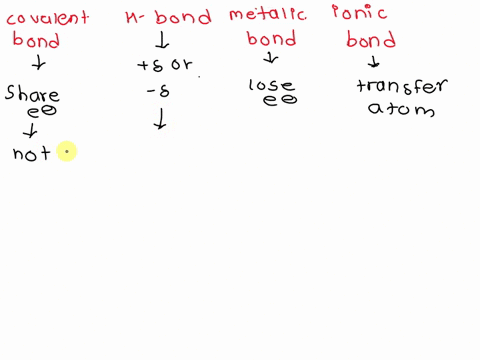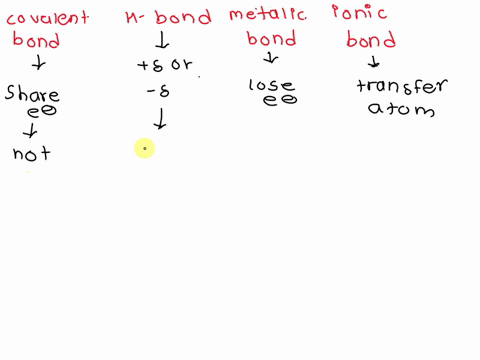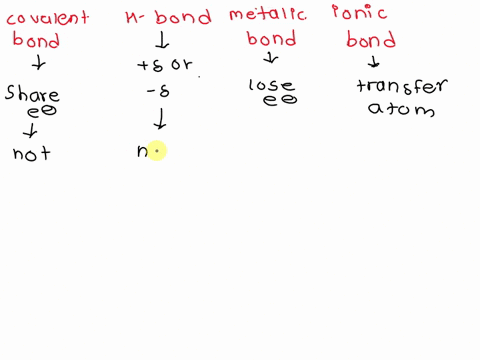
Source Image: numerade.com
Download Image
compare and contrast Covalent Bonds and Ionic Bonds [classic] | Creately
B: Ionic bonding between sodium and chlorine, forming a lattice structure. Ionic compounds contain both cations and anions in a ratio that results in zero electrical charge. As shown in Figure \(\PageIndex2\) , the strength of the interaction is proportional to the magnitude of the charges and decreases as the distance between the particles
Source Image: creately.com
Download Image
IJMS | Free Full-Text | Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes
(a) only elements in the same row of the periodic table (b) only elements in the same column of the periodic table (c) elements with significant differences in electronegativity (d) elements with
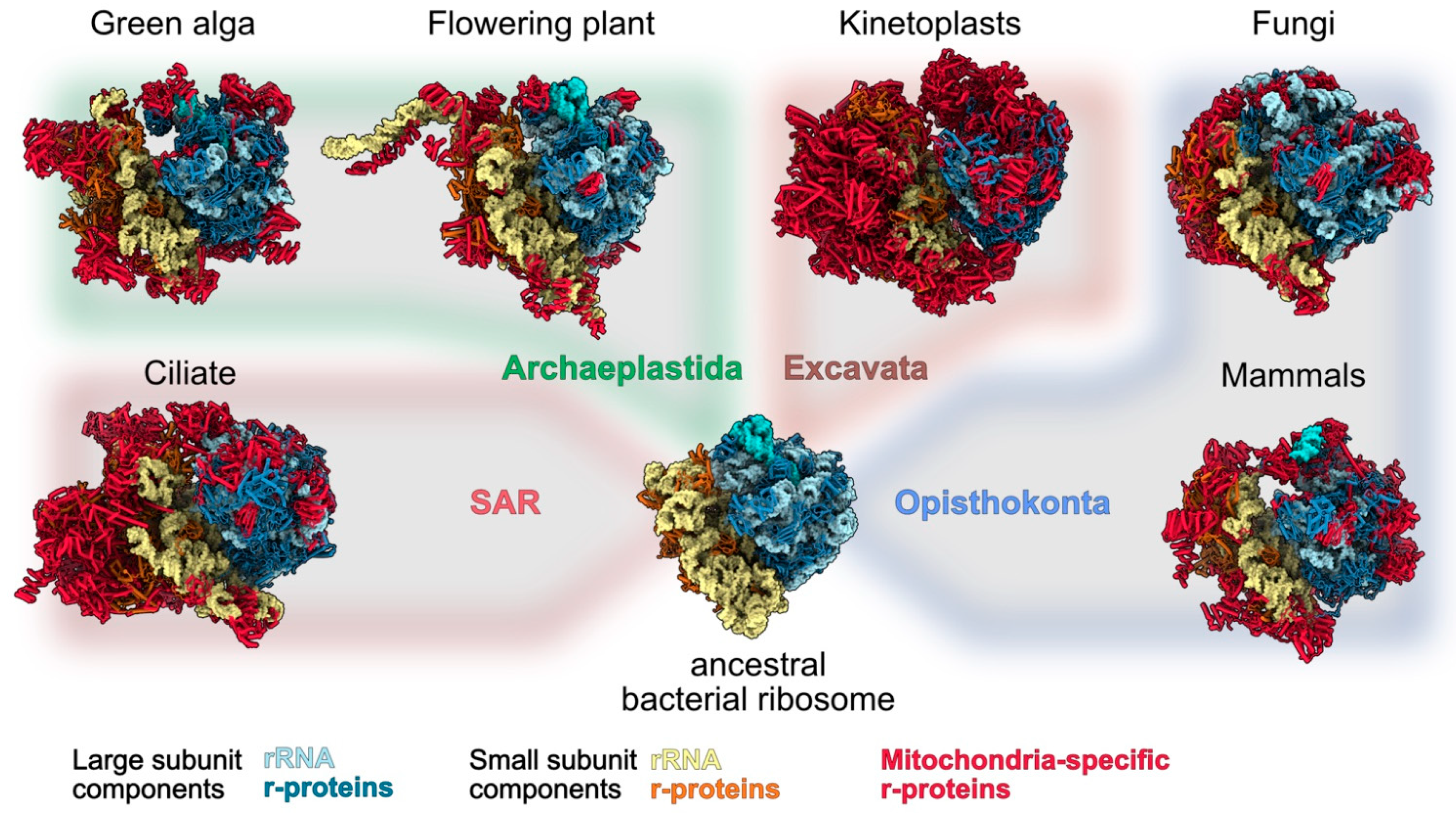
Source Image: mdpi.com
Download Image
SOLUTION: Topic 4 revision hl – Studypool
Ionic compounds form crystal lattices rather than amorphous solids. They have higher enthalpies of fusion and vaporization than molecular compounds. They are hard. They are brittle. They have high melting points and also high boiling points. They conduct electricity but only when they are dissolved in water. … Deevona · 52 · Oct 2 2014 Questions

Source Image: studypool.com
Download Image
Electrical Double Layer Structure in Ionic Liquids and Its Importance for Supercapacitor, Battery, Sensing, and Lubrication Applications | The Journal of Physical Chemistry C
SOLUTION: Topic 4 revision hl – Studypool
3.1 I can compare and contrast the structure of ionic and covalent compounds and give examples of each. 3.2 I can determine the number of atoms in a compound when given the formula. 3.3 I can name ionic compounds when given the formulas. 3.4 I can write formulas for ionic compounds when given the names.
SOLVED: Contrast the structures of ionic compounds and metals. IJMS | Free Full-Text | Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes
B: Ionic bonding between sodium and chlorine, forming a lattice structure. Ionic compounds contain both cations and anions in a ratio that results in zero electrical charge. As shown in Figure \(\PageIndex2\) , the strength of the interaction is proportional to the magnitude of the charges and decreases as the distance between the particles